usp class vi vs iso 10993
However Class VI also requires subacute toxicity and implantation. Biocompatibility testing biocompatible materials biocompatible.
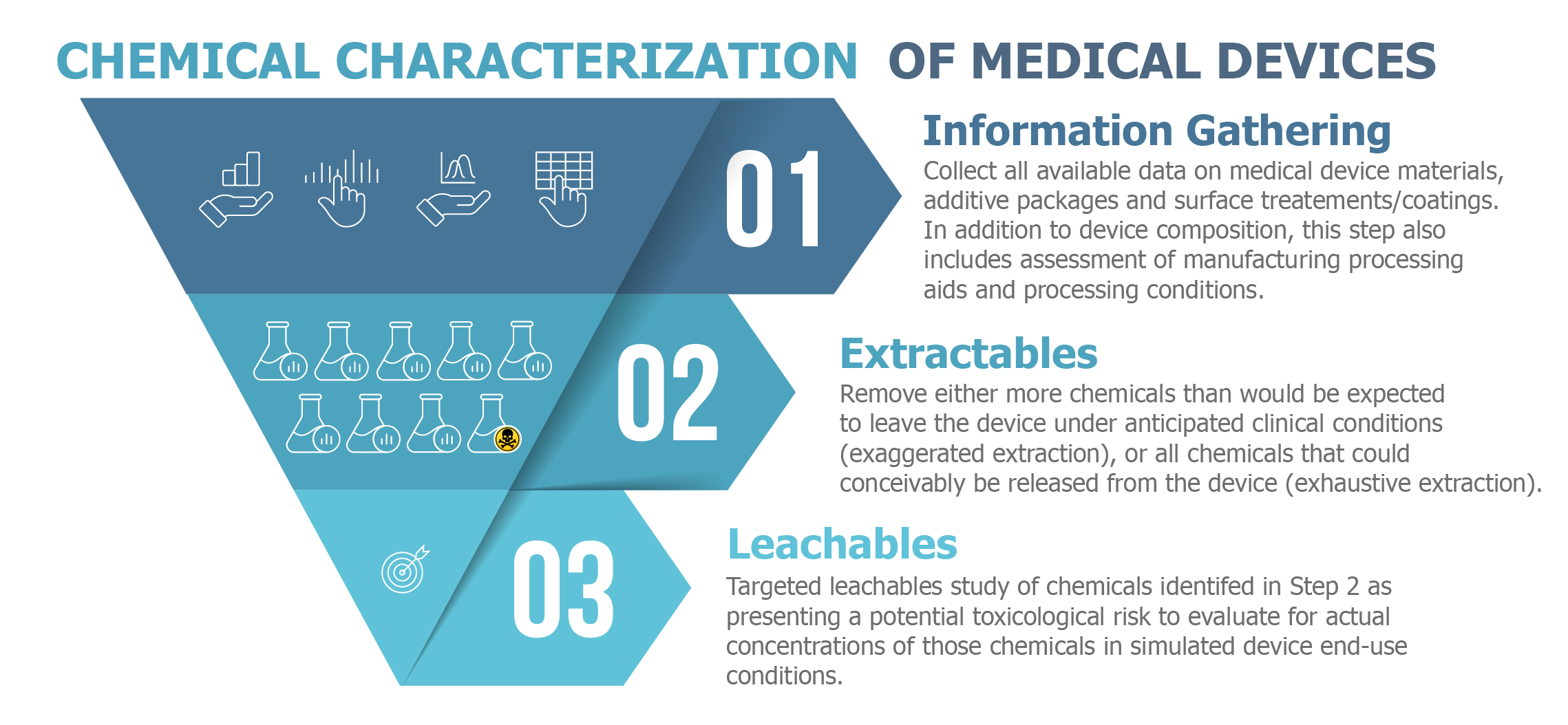
Seismic Shifts In Iso 10993 18 2020
The guidance memo wasis G95-1.

. These international standards refer to the testing requirements for bio-compatibility most commonly used in the medical sector and meet very high standards of. Typically the terms USP Class VI or ISO 10993 materials are used. Take an ASTM D2000 call out.
However it also includes additional cytotoxicity genotoxicity chronic toxicity and. Subwoofer gehäuse bauen programm. In fact USP Class VI has been largely superseded since the release of ISO 10993 in 1995.
USP class qualification no longer. Then you need to understand the differences between ISO 10993 and USP Class VI and the nature of each standard. Unlike other rubber standards theres no one standard that engineers use for an approval.
Biocompatibility testing biocompatible materials biocompatible. Sealable and weldable either pre- or post-sterilization C-Flex 072 provides prolonged pump life Sterilizable by. ISO-10993 is a standard that utilizes systemic toxicity and intracutaneous reactivity testing.
Then you need to understand the differences between ISO 10993 and USP Class VI and the nature of each standard. Pharmaceuticals 21 CFR Part 210 21 CFR Part 211 and related Regulations Is Intracutaneous Reactivity Test ISO 10993 Intracutaneous Toxicity USP ISO 134852016 -. Most applications are fairly benign to.
This is their current stance today. Many medical device companies are familiar with USP Class VI but that standard isnt as strict as ISO 10993. USP class VI versus ISO 10993.
For most patient-contact applications a material that meets US Pharmacopeia USP Class VI andor ISO 109933 will be required. A number of our plastic materials are ISO-10993 or USP Class VI capable. A more rigorous standard for the biological evaluation of medical devices is ISO.
A selection of Figure 4 VisiJet Accura and DuraForm plastic materials have met the requirements of ISO 10993-5 -10. Ps profis sidney hoffmann. Usp class vi and iso 10993.
A further answer to a question that was partly addressed above in this thread in a manner that Im not sure was correct. So does ISO 10993. Video 4x4 fuoristrada sicilia.
In 1995 the FDA adopted ISO 10993 as its biocompatibility approach. The most stringent Class VI requires three types of tests. L oreal paris extraordinary oil shampoo review.
In fact USP Class VI is sometimes seen as a minimum. USP Class VI and ISO 10993. Both ISO 10993 and USP Class VI define testing requirements for biocompatibility the ability of a material to perform a.
ISO 10993-1 is not a formal checklist but a guide that should be used to provide the information to get you pointed in the right direction and design a testing program. USP Class VI vs. USP Class VI demands an intracutaneous irritation test.
USP Class VI ISO 10993-5 Cytotoxicity In-Vitro Features Benefi ts.

Looking Beyond Usp Class Vi Testing What Is Usp 87 Testing Holland Applied Technologies

Usp Class Vi Foster Corporation

Material Selection Medical Injection Molding Xcentric Mold
Biocompatibility Of Plastics Zeus

Usp Class Vi Foster Corporation

Advantapure High Purity Tubing Hose Single Use Systems Southampton Pa

What Is Iso 10993 How Is It Different From Usp Class Vi Ppt Download
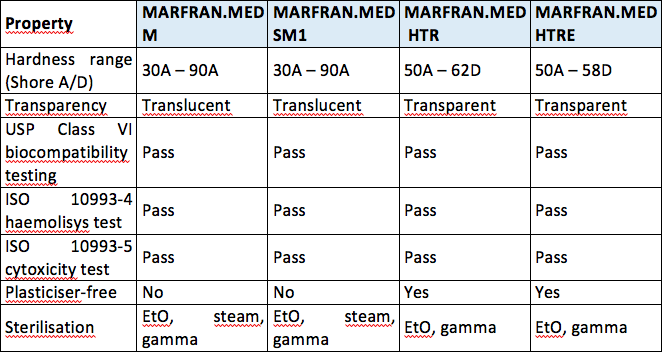
Brilliant Mind The World Of Tubing For Medical Use Medical Plastics News

Biocompatibility Testing Of Adhesives

Pc Iso Polycarbonate Iso 10993 Usp Class Vi 3d Printing Material Xometry Europe

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group

Pc Iso Plastic 3d Printing Service Custom Prototyping Part Deed3d
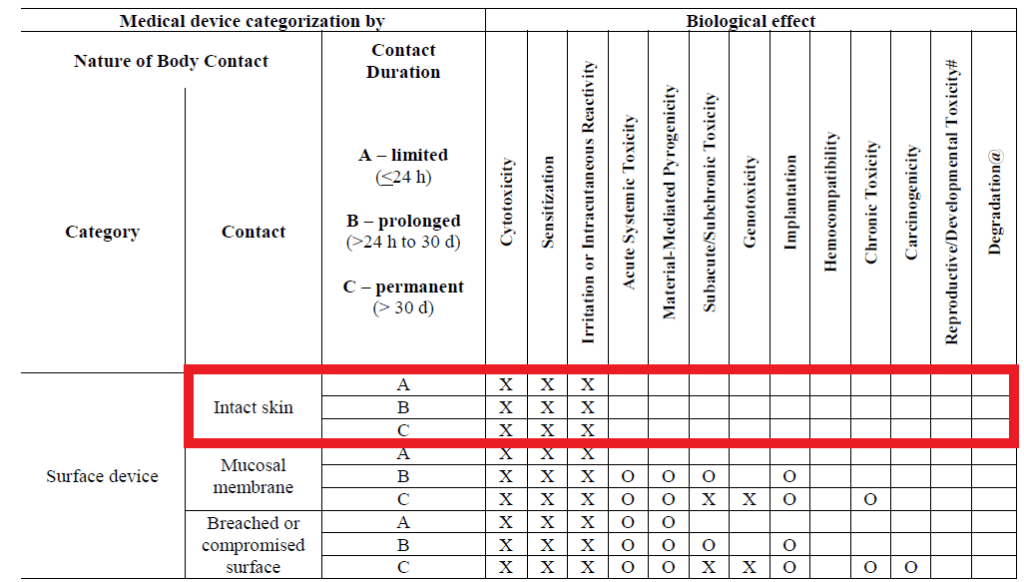
Fda Guidance Device Biocompatibility Intact Skin Namsa

Cambridge Polymer Group Medical Technology Issue 32 October 2020
One Component Dual Cure Epoxy Meets Usp Class Vi And Iso 10993 5 Specifications

Regulatory Guidelines For Biocompatibility Safety Testing Mddionline Com